The unit
Bergamo, a company within the Amgen group since 2011, has its factory located in Taboão da Serra (SP), with areas of Production, Quality, Supply Chain, Maintenance, EHSS (Environment, Health, Safety and Sustainability) and Research & Development.
Our current manufacturing plant was inaugurated in 1992, and in 2009 it already had the oncological injectables area in full operation. After the acquisition by Amgen, approximately R$ 50 million was invested in Bergamo Business Unit, aimed to put into practice a robust portfolio and pipeline planning to enhance the partnership between the companies and guarantee Amgen's global manufacturing best practices, aligned with the requirements of international regulatory agencies, in order to optimize the quality and innovation competencies that already characterized our laboratory.
These investments contributed to the expansion of the factory's production capacity, focus on research, development and production of medicines, launch of new products and the consequent expansion of our presence in health institutions and in patient's lives.
Our factory control systems comply with the requirements of Manufacturing Best Practices demanded by regulatory agencies. We have a production of approximately 2.2 million units distributed in 2021.
Lean Transformation
Bergamo has an Operational Excellence area that supports teams in the implementation of the Lean Transformation program. This initiative aims the constant search for efficiency, quality promotion, increasing productivity and reducing deadlines, waste and costs, and is based on the concepts of Lean Manufacturing.
Our Lean Transformation program is divided into 4 stages: Stability, Flow, Pull and Integration, in other words, this implementation in our operations takes place in a phased manner. At each step incorporated - with implemented practices, established metrics and satisfactory results -, we are evaluated by the global Operational Excellence team, which certifies our consolidation in that particular phase of the program.
In 2018, we obtained the Stability and Flow certification. More recently, we have incorporated the Pull Manufacturing System in our plant, which essentially changes the way we plan our production, as well as the flow of information and materials in our factory. With this new model, which started to be planned in 2019, we are no longer producing based on the sales forecast, but based on stock replacement of sold products. Our production is planned according to the real demand for medicines, reflected by costumer consumption.
This makes us the first Amgen operations site to implement the pull system across all manufacturing processes. We are a benchmark for other Amgen plants around the world.

We develop, produce and commercialize medicines for the treatment of cancer, infectious diseases and other conditions.

We are one of the main generic and similar oncological factories in Brazil belonging to a multinational group.

Main areas of investment: generic and similar drugs for Onco-Hematology and for the Hospital segment.
Evolution of our manufacturing operations in line with the Amgen group
- Portfolio Review to enhance the synergy between Amgen's and Bergamo's products.
- Beginning of corporate audits.
- Investment plan for plant renovation – ~R$50 million.
- Beginning of Lean Transformation
- Consolidation of the Bergamo Business Unit within Amgen Brazil's structure.
- Definition of Bergamo's strategic plan.
- Renewal of packaging lines.
- Lean Transformation: certification of the Stability and Flow phases with Amgen Global.
- Implementation of a third production shift.
- Installation and start of operation of the second Lyophilizer.
- Lean Transformation: Start of the implementation of the Pull System.
- Start of laboratories renovation.
- Consolidation of the plant in two buildings.
- Beginning of the Bergamo products exportation initiative.
- Approval of innovation aimed at product storage in hematology.
- EvolutionTimeline6_Item1
- EvolutionTimeline6_Item2
- EvolutionTimeline6_Item3
- EvolutionTimeline6_Item4
OpenDoorsProgram_evolucao_operacoes

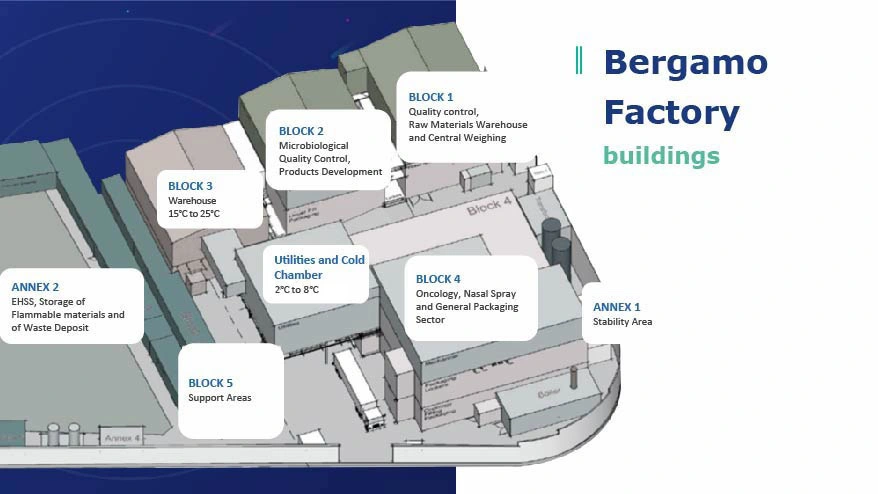
Discovering the Factory
Our plant is divided and organized into several blocks and buildings, in which our R&D, Quality, Packaging and Production teams, among others, are distributed.
Our entire manufacturing plant has EHSS (Environment, Health, Safety and Sustainability) support and monitoring. The department works to maintain a safe and sustainable environment, through constant monitoring of health and safety conditions at work and proper waste disposal, so that we can provide all employees with quality of life at work and preserve the environment. Always operating in line with the global guidelines of the Amgen group and its Sustainability Plan, which includes goals to be achieved by 2027.

The department, in addition to ensuring compliance with all legislation regarding the monitoring of the health of our employees (factory and office), in recent years has been paying special attention to actions against the coronavirus, mainly due to those who remained working in person during the pandemic, performing daily temperature measurements and digital contact mapping, using signs, providing information and all the support, including related to mental health.
Bergamo's Open Doors Program*
(*) for 2022 we have planned visits for the following months: August, September and November
Every month, we receive between five and six guests at our factory, generally professionals who are part of institutions that the Amgen Brazil group maintains contact with, for a visit so they can see our facilities and some of the processes. These visitors are invited by our Field Force staffs.
The Open Doors Program also features a presentation in which visitors receive information about biotechnology, Amgen and Bergamo's history and values, products and therapeutic areas in which we operate, research & development, quality control and standards, pharmacovigilance notions and information on how a pharmaceutical industry works. They are then invited to take a tour by some areas of our facilities.
If you are interested in participating in the program, contact our Customer Service Center to request the contact of a Bergamo representative in your region.